MAKING A DIFFERENCE IN ADC
WITH GEMINIMAB
At Syndivia, we are dedicated to advancing treatment options for cancer patients through our innovative GeminiMab Technology. This platform enables the creation of site-specific antibody-drug conjugates (ADCs) in the hinge region, ensuring optimal drug-to-antibody ratios (DAR), including the unique site-specific DAR1. Our approach leverages these targeted ADCs' distinct properties to significantly enhance antitumor efficacy, offering a powerful and well-tolerated therapeutic option for cancer patients.
THE SCIENCE
GEMINIMAB ADC PLATFORM
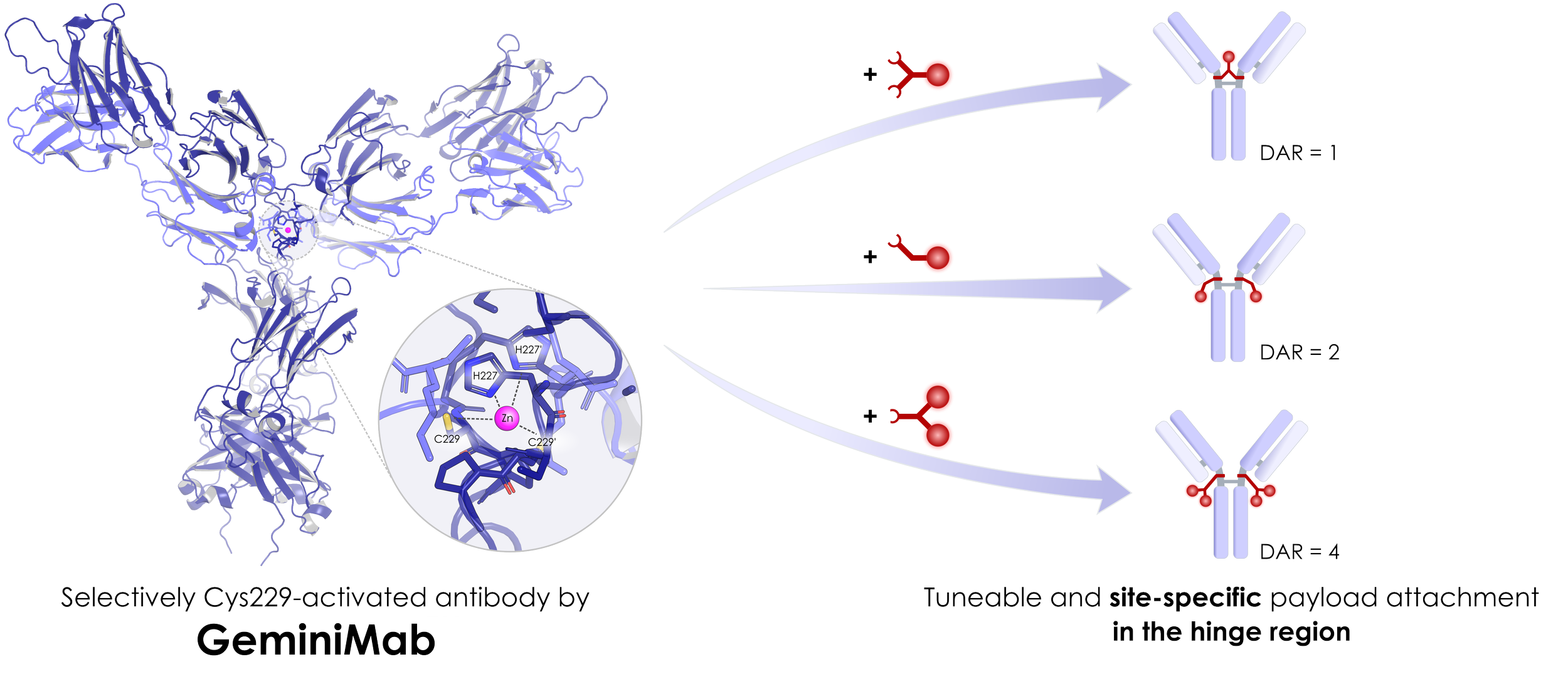
Bringing site-specificity to the hinge: GeminiMab is the first and only platform that allows for hinge-region site-specific conjugation of native antibodies. It enables unprecedented simplicity and scope in creating efficient and well-tolerated antibody conjugates with optimal drug-to-antibody ratios (DAR), including unique DAR1, DAR2, and DAR4 hinge region site-specific ADCs.
GeminiMab eliminates the need for enzymes, antibody sequence modification, or specific pretargeting units to achieve site specificity. The resulting conjugates preserve the native glycosylation profile, and maintain antibody flexibility and integrity, while ensuring the covalent linkage between antibody chains. The conjugation site is located in the safest and most clinically validated antibody hinge region, naturally concealing payload hydrophobicity and immunogenicity without requiring additional modifications or tweaks of the payload.
OPTIMAL DAR & CONJUGATION SITE
Syndivia leverages GeminiMab Technology to develop a proprietary pipeline of ADC candidates with payloads conjugated at the optimal drug-to-antibody ratios (DAR) site-specifically within the most clinically validated and efficient site on the antibody – the hinge region. This allows for the highest dosing without compromising safety. Achieved through improved tumor penetration and enhanced therapeutic efficacy, GeminiMab stands apart from other available ADC technologies.
Accessing optimal DARwith GeminiMab
-
1. Dattler D., Schlimpen F., Semenchenko C., Koniev O. & Kolodych S. Site-specific conjugation on Cys229 of the hinge region of native antibodies improves pharmacological properties of ADCs (June 2024).
2. Kurian, A. S. N., Gurukandure, A., Dovgan, I., Kolodych, S. & Easley, C. J. Thermofluorimetric Analysis (TFA) using Probes with Flexible Spacers: Application to Direct Antibody Sensing and to Antibody–Oligonucleotide (AbO) Conjugate Valency Monitoring. Anal Chem 95, 11680–11686 (2023).
3. Lehot, V. et al. Reinvestigation of the Automated Synthesis of Stoichiometrically Conjugated Antibodies to Access High Molecular Weight Payloads and Multiplexed Conjugation via an In-Solution Trans-Tagging Process. ACS Omega 8, 40508–40516 (2023).
4. Lehot, V. et al. Non-specific interactions of antibody-oligonucleotide conjugates with living cells. Sci Rep 11, 5881 (2021).
5. Dovgan, I. et al. Automated linkage of proteins and payloads producing monodisperse conjugates. Chem Sci 11, 1210–1215 (2020).
6. Dovgan, I. et al. On the use of DNA as a linker in antibody-drug conjugates: synthesis, stability and in vitro potency. Sci Rep 10, 7691 (2020).
7. Dovgan, I., Koniev, O., Kolodych, S. & Wagner, A. Antibody–Oligonucleotide Conjugates as Therapeutic, Imaging, and Detection Agents. Bioconjug Chem 30, 2483–2501 (2019).
8. Riomet, M. et al. Design and Synthesis of Iminosydnones for Fast Click and Release Reactions with Cycloalkynes. Chemistry – A European Journal 24, 8535–8541 (2018).
9. Koniev, O. et al. Reduction–rebridging strategy for the preparation of ADPN-based antibody–drug conjugates. Medchemcomm 9, 827–830 (2018).
10. Renoux, B. et al. Targeting the tumour microenvironment with an enzyme-responsive drug delivery system for the efficient therapy of breast and pancreatic cancers. Chem Sci 8, 3427–3433 (2017).
11. Dovgan, I. et al. Acyl Fluorides: Fast, Efficient, and Versatile Lysine-Based Protein Conjugation via Plug-and-Play Strategy. Bioconjug Chem 28, 1452–1457 (2017).
12. Kolodych, S. et al. Development and evaluation of β-galactosidase-sensitive antibody-drug conjugates. Eur J Med Chem 142, 376–382 (2017).
13. Bernard, S. et al. Bioorthogonal Click and Release Reaction of Iminosydnones with Cycloalkynes. Angewandte Chemie International Edition 56, 15612–15616 (2017).
14. Al‐Shuaeeb, R. A. A. et al. Palladium‐Catalyzed Chemoselective and Biocompatible Functionalization of Cysteine‐Containing Molecules at Room Temperature. Chemistry – A European Journal 22, 11365–11370 (2016).
15. Dovgan, I., Kolodych, S., Koniev, O. & Wagner, A. 2-(Maleimidomethyl)-1,3-Dioxanes (MD): a Serum-Stable Self-hydrolysable Hydrophilic Alternative to Classical Maleimide Conjugation. Sci Rep 6, 30835 (2016).
16. Liu, H. et al. Ultrafast Click Chemistry with Fluorosydnones. Angewandte Chemie International Edition 55, 12073–12077 (2016).
17. Kolodych, S. et al. CBTF: New Amine-to-Thiol Coupling Reagent for Preparation of Antibody Conjugates with Increased Plasma Stability. Bioconjug Chem 26, 197–200 (2015).
18. Koniev, O. et al. MAPN: First-in-Class Reagent for Kinetically Resolved Thiol-to-Thiol Conjugation. Bioconjug Chem 26, 1863–1867 (2015).
19. Koniev, O. & Wagner, A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev. 44, 5495–5551 (2015).
-
Syndivia actively protects its innovative products and technologies, especially focusing on the DAR1 antibody-drug conjugate platform and conjugation technologies. We achieve this by patenting our products and processes and obtaining exclusive licenses from top academic and research institutions.
Our strategy is built on using our in-house expertise, continuously innovating, and strategically licensing technologies to maintain our unique market position. We strive to secure patents both in our home country and internationally, always looking to expand our intellectual property portfolio.
Our IP portfolio includes five main patent families, specifically targeting our DAR1 platform and conjugation technologies. This encompasses patents we own ourselves and those we have exclusively licensed from research institutions, providing strong protection for our core technologies.

PIPELINE

OUR TEAM
MANAGEMENT TEAM
SCIENTIFIC ADVISORY BOARD

NEWS & PRESS

CONTACT
CAREERS
We invite applications from seasoned professionals in chemistry, biology, and bioconjugation. We are committed to diversity and welcome candidates of any gender, race, religion, color, sexual orientation, disability status, and marital or parental status. If you’re passionate about advancing next-generation therapeutics, we’d love to hear from you.
OUR SITE
Syndivia
8 allée Gaspard Monge,
67083 Strasbourg, France
Email: enquiry@syndivia.com